Properties of acid and bases worksheet – Embark on a scientific odyssey with our properties of acids and bases worksheet, an indispensable resource that unravels the fascinating world of these fundamental chemical entities. Dive into their characteristic properties, explore their real-world applications, and master the art of identifying and comparing acids and bases.
From the pH scale to neutralization reactions, this worksheet empowers you with a comprehensive understanding of the behavior and significance of acids and bases in our world.
Properties of Acids
Acids are chemical substances that exhibit characteristic properties, including:
- Sour taste
- Corrosive to skin and metals
- React with bases to form salts
- Turn blue litmus paper red
Common examples of acids include:
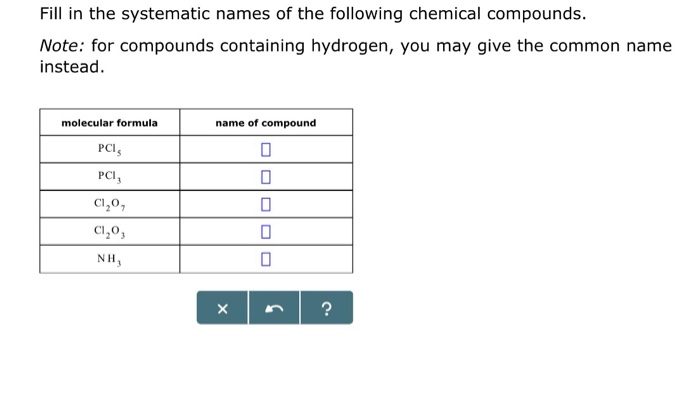
- Hydrochloric acid (HCl)
- Sulfuric acid (H 2SO 4)
- Nitric acid (HNO 3)
- Acetic acid (CH 3COOH)
Acids have a pH range of less than 7.
Properties of Bases
Bases, also known as alkalis, exhibit distinctive properties:
- Bitter taste
- Slippery to the touch
- React with acids to form salts
- Turn red litmus paper blue
Common examples of bases include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH) 2)
- Ammonia (NH 3)
Bases have a pH range of greater than 7.
Comparing Acids and Bases: Properties Of Acid And Bases Worksheet
| Property | Acid | Base |
|---|---|---|
| Taste | Sour | Bitter |
| Feel | Corrosive | Slippery |
| Reaction with metals | Produces hydrogen gas | No reaction |
| Reaction with litmus paper | Turns blue litmus red | Turns red litmus blue |
| pH range | < 7 | > 7 |
Acids and bases are opposites in terms of their pH values and reactivity. Acids are proton donors, while bases are proton acceptors.
Neutralization Reactions
Neutralization reactions occur when an acid and a base react in stoichiometric proportions to form a salt and water.
Worksheet on Neutralization Reactions
Procedures:
- Measure out a known volume of an acid solution.
- Add a known volume of a base solution slowly, while stirring constantly.
- Monitor the pH of the solution using a pH meter or litmus paper.
- Continue adding the base until the solution reaches a neutral pH of 7.
Calculations:
- Calculate the moles of acid used.
- Calculate the moles of base used.
- Determine the limiting reagent.
- Calculate the theoretical yield of the salt.
- Compare the theoretical yield to the actual yield obtained.
Applications of Acids and Bases
Acids:
- Used in batteries
- Used in food preservation
- Used in manufacturing fertilizers
- Used in chemical reactions
Bases:
- Used in cleaning products
- Used in manufacturing soaps and detergents
- Used in neutralizing acids
- Used in medical applications
Acids and bases are essential substances in everyday life, with numerous applications in various industries and fields.
User Queries
What are the key properties of acids?
Acids are characterized by their sour taste, ability to react with metals to produce hydrogen gas, and ability to turn blue litmus paper red.
What are some common examples of bases?
Common bases include sodium hydroxide, potassium hydroxide, and ammonia.
How can I identify an acid or base using a pH indicator?
Acids have a pH below 7, while bases have a pH above 7. A pH indicator, such as litmus paper, can be used to determine the pH of a solution and identify whether it is acidic or basic.