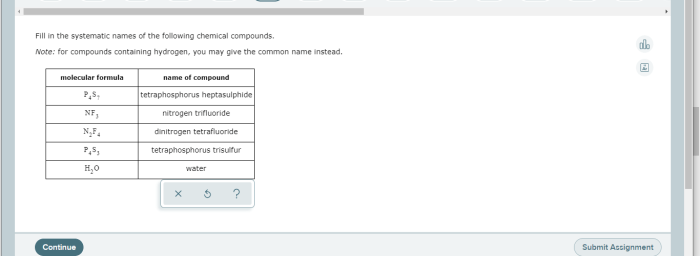
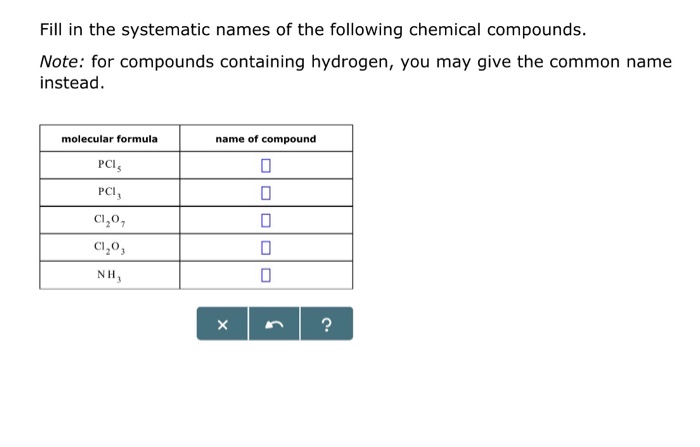
Fill in the systematic names of the following chemical compounds sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This definitive guide delves into the fascinating realm of chemical nomenclature, providing a comprehensive overview of the rules, conventions, and intricacies involved in naming inorganic, organic, coordination, polymer, and biological compounds.
Embark on a journey of discovery as we unravel the secrets of systematic nomenclature, empowering you to navigate the complex world of chemistry with precision and confidence.
Throughout this guide, you will encounter a wealth of knowledge and insights that will illuminate the fundamental principles of chemical nomenclature. We will explore the IUPAC guidelines that govern the naming of organic compounds, deciphering the intricate language of functional groups and their impact on molecular identity.
Delve into the realm of inorganic nomenclature, where prefixes and suffixes dance in harmony to convey the elemental composition and oxidation states of compounds. Discover the intricacies of coordination compound nomenclature, unraveling the mysteries of ligands and metal complexes. Explore the systematic naming of polymers, unraveling the secrets of their structure and properties.
Finally, venture into the realm of biological molecule nomenclature, where abbreviations and codes hold the key to unlocking the identities of proteins, carbohydrates, lipids, and nucleic acids.
Systematic Nomenclature of Inorganic Compounds
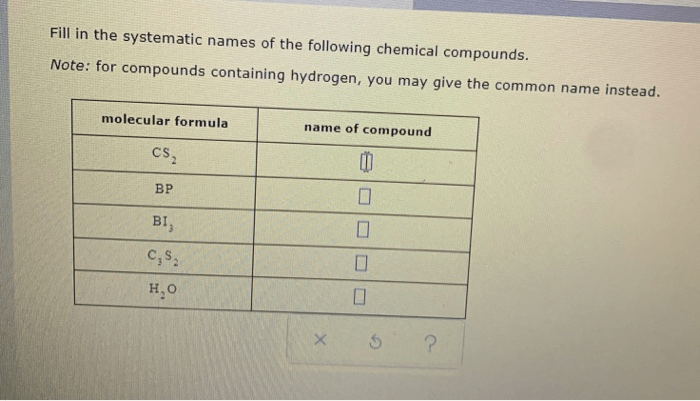
Systematic nomenclature for inorganic compounds follows specific rules and conventions to assign unique and descriptive names to chemical substances. These rules aim to provide a consistent and standardized method for naming inorganic compounds, facilitating communication and understanding among chemists.
The International Union of Pure and Applied Chemistry (IUPAC) has established guidelines for systematic inorganic nomenclature, which include the following principles:
- The name of a simple inorganic compound typically consists of the name of the cation followed by the name of the anion.
- The cation is named first, followed by the anion.
- The name of the cation is typically derived from the element’s name, while the name of the anion is derived from the element’s name with the suffix “-ide”.
- For polyatomic ions, the name of the anion is derived from the element’s name with the suffix “-ate” or “-ite”.
- The oxidation state of the metal in the cation is indicated using Roman numerals in parentheses after the metal’s name.
Prefixes and Suffixes in Inorganic Nomenclature
Inorganic nomenclature also employs prefixes and suffixes to indicate the number of atoms or ions present in a compound.
| Prefix | Meaning |
|---|---|
| mono- | one |
| di- | two |
| tri- | three |
| tetra- | four |
| penta- | five |
| hexa- | six |
For anions, the following suffixes are used to indicate the charge:
| Suffix | Meaning |
|---|---|
| -ide | -1 charge |
| -ate | -2 charge |
| -ite | -3 charge |
Examples of Systematic Names for Simple Inorganic Compounds
- NaCl: sodium chloride
- MgO: magnesium oxide
- Fe2O3: iron(III) oxide
- CuSO4: copper(II) sulfate
- NH4Cl: ammonium chloride
Systematic Nomenclature of Organic Compounds
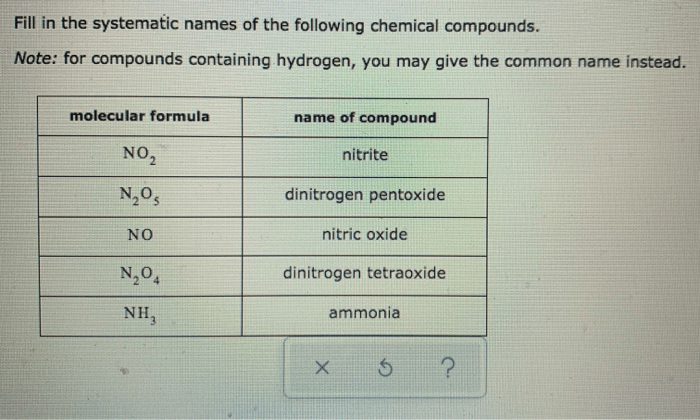
The systematic nomenclature of organic compounds follows a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC). These rules aim to provide a consistent and unambiguous method for naming organic compounds, facilitating communication and understanding among chemists.
The IUPAC system for naming organic compounds is based on the following principles:
- The name of an organic compound is derived from the name of the parent hydrocarbon.
- The parent hydrocarbon is the longest continuous chain of carbon atoms in the molecule.
- The name of the parent hydrocarbon is modified by prefixes and suffixes to indicate the presence of functional groups.
- Functional groups are atoms or groups of atoms that give organic compounds their characteristic chemical properties.
Classes of Organic Compounds and Their Characteristic Functional Groups
Organic compounds are classified into different classes based on the functional groups they contain. Some common classes of organic compounds and their characteristic functional groups include:
- Alkanes: contain only carbon and hydrogen atoms, and have the general formula CnH2n+2.
- Alkenes: contain at least one carbon-carbon double bond, and have the general formula CnH2n.
- Alkynes: contain at least one carbon-carbon triple bond, and have the general formula CnH2n-2.
- Alcohols: contain a hydroxyl group (-OH), and have the general formula CnH2n+1OH.
- Aldehydes: contain a carbonyl group (-CHO), and have the general formula CnH2n+1CHO.
- Ketones: contain a carbonyl group (-CO-), and have the general formula CnH2n+1CO.
- Carboxylic acids: contain a carboxyl group (-COOH), and have the general formula CnH2n+1COOH.
Examples of Systematic Names for Common Organic Compounds, Fill in the systematic names of the following chemical compounds
- CH4: methane
- C2H4: ethene
- C3H6: propene
- CH3OH: methanol
- CH3CHO: ethanal
- CH3COCH3: propanone
- CH3COOH: ethanoic acid
Nomenclature of Coordination Compounds
Coordination compounds are molecular entities that contain a metal center surrounded by ligands. The nomenclature of coordination compounds follows a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
The name of a coordination compound consists of the following parts:
- The name of the metal center
- The names of the ligands
- The oxidation state of the metal center (indicated in Roman numerals)
Naming Ligands
Ligands are named according to the following rules:
- Anionic ligands are named by replacing the ending “-ide” of the anion name with “-o”.
- Neutral ligands are named by adding the suffix “-amine” to the name of the parent hydrocarbon.
- Cationic ligands are named by adding the suffix “-ium” to the name of the parent hydrocarbon.
Examples of Systematic Names for Coordination Compounds
- [Co(NH3)6]Cl3: hexamminecobalt(III) chloride
- [Fe(CN)6]4-: hexacyanoferrate(II) ion
- [Pt(en)2Cl2]: dichloro(ethylenediamine)platinum(II)
Nomenclature of Polymers
Polymers are large molecules composed of repeating structural units called monomers. The nomenclature of polymers follows a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
The name of a polymer consists of the following parts:
- The prefix “poly-“
- The name of the monomer
- The suffix “-ane”
Examples of Systematic Names for Common Polymers
- (C2H4)n: polyethylene
- (C3H6)n: polypropylene
- (C4H8)n: polybutene
- (C6H5CH=CH2)n: polystyrene
- (C8H8O4)n: polyethylene terephthalate (PET)
Nomenclature of Biological Molecules: Fill In The Systematic Names Of The Following Chemical Compounds
Biological molecules are complex organic molecules that are essential for life. The nomenclature of biological molecules follows a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC) and other international organizations.
The nomenclature of biological molecules is based on the following principles:
- The name of a biological molecule is derived from its structure or function.
- The name of a biological molecule is typically abbreviated using a code or symbol.
Examples of Systematic Names for Common Biological Molecules
- Deoxyribonucleic acid (DNA)
- Ribonucleic acid (RNA)
- Protein
- Carbohydrate
- Lipid
Key Questions Answered
What is the purpose of systematic nomenclature in chemistry?
Systematic nomenclature provides a standardized and unambiguous system for naming chemical compounds, enabling scientists to communicate complex chemical structures clearly and accurately.
How does systematic nomenclature help in understanding chemical properties?
Systematic names convey information about a compound’s composition, structure, and functional groups, providing valuable insights into its chemical properties and reactivity.
What are the key principles of IUPAC nomenclature?
IUPAC nomenclature follows specific rules and conventions, including the use of prefixes, suffixes, and parent chains, to assign systematic names to organic compounds based on their structure and functional groups.