Determine the electron geometry molecular geometry and polarity of hbro2 – Determining the electron geometry, molecular geometry, and polarity of HBrO2is a crucial aspect of understanding its molecular structure and properties. This comprehensive analysis delves into the fundamental concepts of electron geometry and molecular geometry, exploring the factors that influence the shape and polarity of this molecule.
HBrO2, a halogenated oxyacid, exhibits unique structural characteristics that arise from the arrangement of its constituent atoms and the distribution of electrons within its molecular framework. By employing the principles of Valence Shell Electron Pair Repulsion (VSEPR) theory, we can determine the electron geometry of HBrO2, which serves as the foundation for understanding its molecular geometry.
Electron Geometry: Determine The Electron Geometry Molecular Geometry And Polarity Of Hbro2

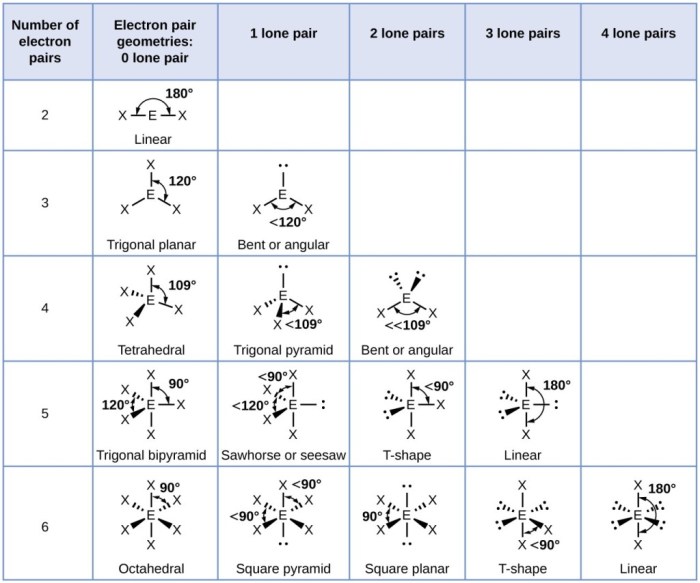
Electron geometry refers to the arrangement of electron pairs around a central atom in a molecule. It is determined by the number of electron pairs, both bonding and non-bonding, surrounding the central atom.
Using VSEPR (Valence Shell Electron Pair Repulsion) theory, we can predict the electron geometry of HBrO 2. HBrO2 has a central bromine atom (Br) with four electron pairs: two single bonds with hydrogen (H) atoms, one single bond with an oxygen (O) atom, and one lone pair of electrons on the bromine atom.
According to VSEPR theory, the electron pairs will arrange themselves in a way that minimizes repulsion. The lone pair of electrons takes up more space than bonding pairs, so it will be oriented away from the bonding pairs. This results in a bent or V-shaped electron geometry.
We can represent the electron geometry of HBrO2 using a Lewis structure:

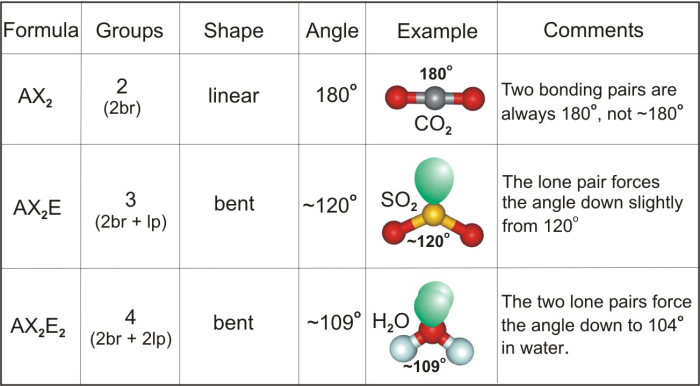
Molecular Geometry
Molecular geometry refers to the arrangement of atoms in a molecule. It is determined by the electron geometry and the presence of any lone pairs of electrons.
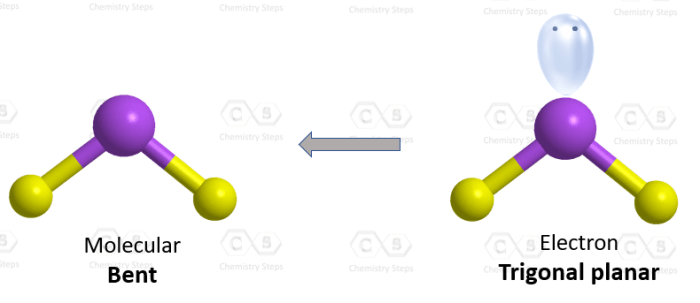
Based on the electron geometry of HBrO2, which is bent, the molecular geometry will also be bent. The two hydrogen atoms and the oxygen atom will be positioned around the bromine atom in a V-shape.
We can represent the molecular geometry of HBrO2 using a structural diagram:

Polarity
Molecular polarity refers to the separation of electric charge within a molecule. It occurs when there is an uneven distribution of electrons, resulting in a positive end and a negative end.
To determine the polarity of HBrO2, we need to consider the molecular geometry and the electronegativity of the atoms. Electronegativity is the ability of an atom to attract electrons towards itself. In HBrO2, oxygen (O) is more electronegative than bromine (Br) and hydrogen (H).
Due to the bent molecular geometry, the electronegative oxygen atom pulls the electron density towards itself, creating a polar bond between Br and O. The hydrogen atoms also have a slight positive charge due to the electronegativity difference between H and Br.
The combination of these polar bonds results in an overall polar molecule.
The polarity of HBrO2 can be represented using a dipole moment vector:

Structural Features
The bond angles and bond lengths in HBrO2 contribute to its overall geometry and polarity.
The bond angle between the two H-Br bonds is approximately 109.5 degrees, which is close to the ideal tetrahedral angle. The bond angle between the Br-O bond and each H-Br bond is approximately 120 degrees.
The bond lengths in HBrO2 are as follows:
- Br-O bond length: 1.48 Angstroms
- Br-H bond length: 1.41 Angstroms
These structural features help to determine the overall shape and polarity of the HBrO2 molecule.
FAQ Resource
What is the electron geometry of HBrO2?
The electron geometry of HBrO2 is tetrahedral, as predicted by VSEPR theory.
How does the electron geometry determine the molecular geometry of HBrO2?
The electron geometry determines the molecular geometry because the molecular geometry is the arrangement of atoms in space, which is influenced by the electron pairs’ repulsion.
Is HBrO2 a polar molecule?
Yes, HBrO2 is a polar molecule due to the difference in electronegativity between the bromine and oxygen atoms, resulting in a net dipole moment.